Background
The prevalence of leg ulcers in sickle cell disease (SCD) patients without hydroxyurea (HU) treatment varies significantly (1.5% to 27%) across studies (Diagne 2000, Minniti 2021, Kato 2018), and the causal relationship between HU and leg ulcers remains debatable. Some investigators express concerns about an increased risk in patients with prior ulceration undergoing HU treatment (Kersgard 2001, Chaine 2001, Nzouakou 2011) while others report no influence (Brawley 2008, Cackovic 1998, Suresh 2004).
HU's potential benefits, including increased hemoglobin (Hb) and fetal hemoglobin (HbF) levels, are thought to improve clinical outcomes, potentially reducing leg ulcer occurrence (Eckman 1996). Concerns are mainly based in reports from, myeloproliferative diseases with cases of leg ulcers in older patients without relevant history after prolonged HU exposure (Sirieix 1999).
Methods
The non-interventional prospective cohort study, ESCORT-HU, involved 1906 patients (55% adults) from 62 centres in Germany, Greece, Italy, and France, from January 2009 to March 2019. While the main objectives of ESCORT-HU were fulfilled regarding data collection on common safety concerns associated with the use of HU in SCD patients, more detailed documentation of specific long-term risks associated with HU treatment is required. In response, the non-interventional ESCORT-HU extension study was initiated in 2020 in the same countries aiming to deepen knowledge on low incidence and indeterminate risks, including leg ulcers. Around 2000 patients treated with HU are expected to participate over the 5-year study duration. Patients from the initial ESCORT-HU study could participate in the extension for long-term follow-up data. New patients must meet specific criteria with a focus on patients with leg ulcers.
Results
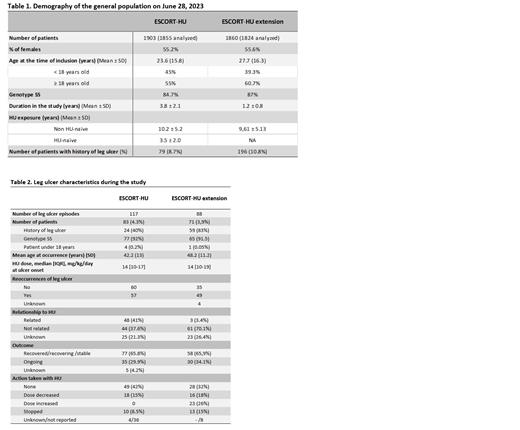
1906 patients were included in ESCORT-HU study, and on June 28, 2023, 1860 patients were included in ESCORT-HU extension study, with 1017 new patients not previously enrolled in the first ESCORT-HU study totalling nearly 3000 patients.
Demographic data and time of HU exposure for both studies are summarized in Table 1.
A total of 117 leg ulcers episodes were reported in 83 patients (4.3%) during ESCORT-HU, while the extension study recorded 88 episodes in 71 patients (3.9%) ( Table 2).
Leg ulcers were mostly reported in patients with the SS genotype (over 90%), and predominantly affect adults. Only five children experienced their first episode from 13.0 to 17.7 years old. However, in the extension study, one child presented an episode of leg ulcer before the age of 10.
Approximately 15% of cases reported at least one risk factors with local trauma, skin dryness and venous insufficiency being the most frequently cited factors.
Reoccurrence of leg ulcers were slightly higher in ESCORT-HU extension (55.7%) compared to ESCORT-HU (48.7%), possibly due to the inclusion of patients with history of leg ulcer in the extension (80% in extension versus 40% in ESCORT-HU). Regarding the relationship with HU, investigators considered 41 % of cases in ESCORT-HU and only 3.4% in the extension related to HU treatment. One plausible reason for confusion among clinicians is that HU can cause skin dryness, a known risk factor associated with leg ulcers. Additionally, it's worth noting that investigators' perceptions may evolve over time.
During the extension study, 10.8% of patients had a history of leg ulcers. Clinicians observed that they were not attributed to HU therapy but rather associated with the underlying disease itself. However, in nearly 25% of cases investigators faced difficulty in determining whether the ulcer was related or not to HU therapy. In over 65% of the patients, the outcome was favourable (recovered and not ongoing), with dose reduction or treatment discontinuation in a minority of patients.
Conclusion
The incidence of leg ulcers in two large cohorts, comprising nearly 3000 patients with prolonged HU treatment, was around 4 %. Leg ulcers pose challenge to continue HU, but the outcome can also be favourable, even with dose increase. Despite a better understanding of the adverse events associated with HU, it remains complex to attribute leg ulcers exclusively to HU. The identification of additional factors or biomarker predisposing to leg ulcers in future large trial is essential.
Disclosures
Etienne-Julan:Addmedica: Membership on an entity's Board of Directors or advisory committees. Bernit:Addmedica: Membership on an entity's Board of Directors or advisory committees. Dimopoulou:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees. Flevari:Addmedica: Membership on an entity's Board of Directors or advisory committees. Cannas:Addmedica: Membership on an entity's Board of Directors or advisory committees. Bartolucci:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bluebird: Consultancy; Roche: Consultancy; Emmaus: Consultancy; GBT: Consultancy; Jazz Pharma: Consultancy; INNOVHEM: Current equity holder in private company; Addmedica: Consultancy, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal